How does the M2 protein of the Influenza A virus bend membranes to produce budding and scission?
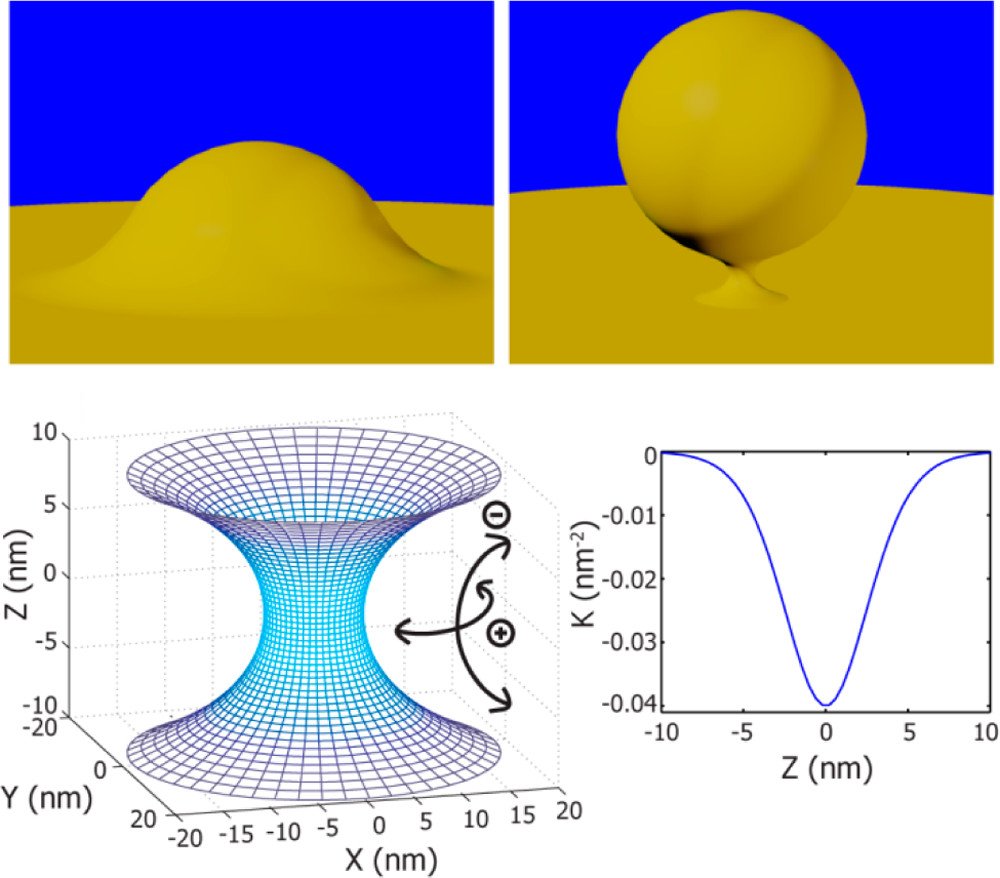 Budding from cells is an essential step in the replication cycle of influenza viruses. Recent work (Rossman J.S., Jing, X., Leser, G.P., Lamb, R.A, Cell 2010, 142, 902) has shown that the influenza A virus M2 protein plays a role in mediating influenza budding and scission, and that this ability is largely conferred by a membrane-associated amphipathic helix in the cytoplasmic portion of the M2 protein. In this work we use small-angle X-ray scattering to conduct a detailed study on the structure and interactions in M2-lipid membrane complexes and use this information to explain how the amphipathic helix imparts M2 with the ability to generate the specific types of membrane curvature necessary for viral budding and scission. We find that M2 proteins actively generate bilayer negative Gaussian membrane curvature (NGC), which geometrically enables blebbing and budding processes. Since the generation of high NGC is geometrically required for successful influenza budding and scission, antagonizing this type of membrane curvature is a viable therapeutic strategy for the next generation of anti-flu drug designs and combination chemotherapies.
Budding from cells is an essential step in the replication cycle of influenza viruses. Recent work (Rossman J.S., Jing, X., Leser, G.P., Lamb, R.A, Cell 2010, 142, 902) has shown that the influenza A virus M2 protein plays a role in mediating influenza budding and scission, and that this ability is largely conferred by a membrane-associated amphipathic helix in the cytoplasmic portion of the M2 protein. In this work we use small-angle X-ray scattering to conduct a detailed study on the structure and interactions in M2-lipid membrane complexes and use this information to explain how the amphipathic helix imparts M2 with the ability to generate the specific types of membrane curvature necessary for viral budding and scission. We find that M2 proteins actively generate bilayer negative Gaussian membrane curvature (NGC), which geometrically enables blebbing and budding processes. Since the generation of high NGC is geometrically required for successful influenza budding and scission, antagonizing this type of membrane curvature is a viable therapeutic strategy for the next generation of anti-flu drug designs and combination chemotherapies.
Others have discussed the ability of proteins/peptides to generate membrane curvature (for example: McMahon, H.T., Gallop, J.L, Nature 2005 438, 590 & Drin, G. Antonny, B. FEBS Lett. 2010 584, 1840). While membrane curvature is a necessary condition for viral budding it is not sufficient – different specific types of curvature are required at different regions of a bud. The neck of a bud is rich in NGC (‘saddle’-shaped curvature), while the top of a bud has positive Gaussian curvature. Our results show that M2 proteins can generate NGC in lipid membranes at the nanoscale, in agreement with recent work on giant vesicles at the micron scale (Rossman et al., Cell, 2010) which described the localization of M2 to the neck of budding virions. Our results show that the structural requirements for scission are more stringent than budding as the neck must constrict to a size much smaller than the virus during ‘pinch off’. We demonstrate that M2 is capable of generating high curvatures comparable to those on a neck, ~10x smaller than a spherical influenza virus. The geometric requirement that M2 generate high NGC places strong constraints on the amino acid composition of the amphipathic helix in the protein. By comparing the amino acid composition of the WT amphipathic helix and the penta-Ala variant helix with antimicrobial peptides whose sequences are also constrained by their ability to generate NGC, we show that M2 budding and scission is highly sensitive to modest reductions in hydrophobicity and NGC generation.